It is partially ionized in its solution. ____ N2 ____ H2 ____ NH3.
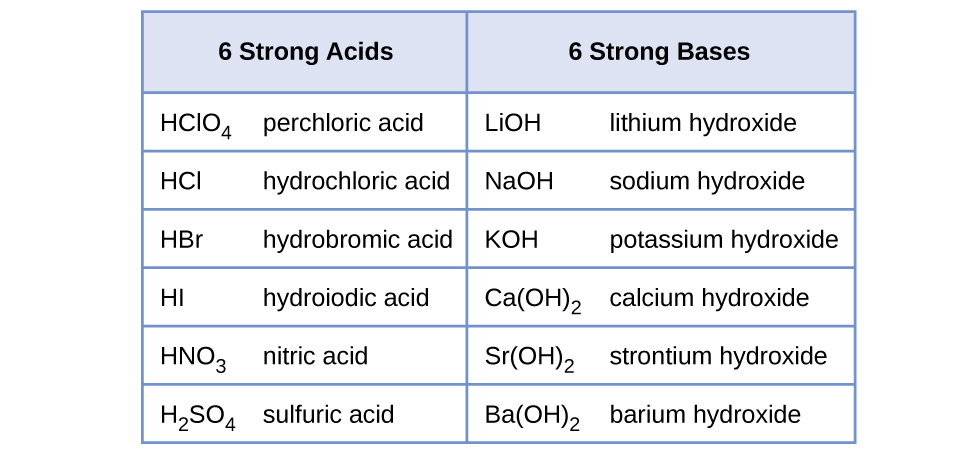
14 3 Relative Strengths Of Acids And Bases Chemistry
A high pH value indicates a high hydroxide concentration.

. A base is any compound that can neutralize an acidTherefore a base should have a hydroxyl group -OH that can be released as a hydroxyl ion. A high pH value indicates a high hydroxide concentration. Chemistry questions and answers.
The temperature increases continually from one point to the next. It is important to note that the conjugate acid of a weak base will almost always be. Which one of the following conditions is always true for a titration of a weak acid with a strong base.
The degrees of disassociation. Weak bases have a pH range of 5-6. Find more answers Ask your question.
1 CH 3 COOH CH 3 COO H. An example of it will be ammonia. 1 on a question.
The equivalence point occurs at a pH less than 7. The larger number of oxygen atoms on the central atom giving it a higher oxidation state also creates a greater release of hydrogen atoms resulting in a stronger acid. A buffer can be prepared by mixing equal amounts of weak acid and a strong base true or false.
After half of the water evaporates the concentration of the solution has ____________. A weak base is a base that ionises or dissociates only partially in water to formOH- ion. KOH which is Potassium hydroxide is a base.
The symbol for the hydroxide ion is _____. It contains hydroxide ions. HA A H 1-1.
Equal volumes of weak acid and strong base are required to reach the equivalence point. What is always true of a weak base. The hydrogen ion will always combine with a water molecule.
The conjugate base of a weak acid is always a strong base. In polyoxy acids the more electronegative central elementS in this caseforms the stronger acid. An increase in heat always produces an increase in temperature.
Correct answer to the question What is always true of a weak base. Balance the following equation. It dissociates in water to form sodium ion and hydroxide ion.
Acetic acid CH 3 COOH is a typical weak acid and it is the ingredient of vinegar. During a phase change the temperature of a substance remains constant. The structure of the acetate ion CH 3 COO is shown below.
Which one of the following conditions is always true for a titration of a weak acid with a strong base. The hydronium ion does not easily break down into water and hydrogen. Some of the original base remains in solution.
It does not dissolve or dissociate in water. Weak bases have a pH range of 5-6. James created a salt water solution by adding a scoop of the solute salt to a beaker of the solvent water.
Main Difference Strong vs Weak Bases. It has been diluted with water. Chemistry questions and answers.
A strong base is a base that ionises or dissociates almost 100 in water to form OH- ion. Since acids are capable of releasing protons H ions these protons can be neutralized by the hydroxyl ions released by the base. The pH at the equivalence point will be 7.
You might be interested in. In a solution of acetic acid the equilibrium concentrations are found to be C. After all the salt dissolves James places the beaker of salt water on a hot plate and begins to heat the solution.
The equivalence point occurs at a pH greater than 7. The equivalence point occurs at a pH equal to 7. A weak acid represented here as HA is one in which the reaction.
On a flat smooth surface such as a. A colored indicator with a pKa less than 7 should be used. An example of a strong base is sodium hydroxide.
Another definition is that a base is referred to as a species that gives out hydroxide OH- ions in aqueous solution it also freely donates electrons and takes protons. A 595 g sample of an acid H2X requires 450 mL of a 0500 M NaOH solution for complete reaction removing both protons. Tangare 24 11 months ago.
The hydronium ion has a positive charge. Equal volumes of weak base and strong acid are required to reach the equivalence point. FiasKO 112 Answer and explanation.
A NaHSeO3 NaHSO3 NaHSO4. Which one of the following conditions is always TRUE for a titration of a weak acid with a strong base. This means that if we add 1 mole of the pure acid HA to water and make the total volume 1 L the equilibrium concentration of the conjugate base A will be smaller often much smaller than 1 ML while that of undissociated HA will be only slightly less than 1 ML.
Describe how a wave is reflected on flat surfaces and curved surfaces. The ionization of a weak base is usually a type of equilibrium process in which a chemical equilibrium is established inside the solution between the concentration of the undissociated base and its constituent ions the conjugate acid and the hydroxide anion. In Chemistry A base is defined as a chemical substance which when dissolved in water gives a slippery feel it has a bitter taste and turns red litmus paper blue.
The hydrogen ion will always combine with a water molecule. What is always true of a weak base. Ionization of Weak Acids.
The hydronium ion does not easily break down into water and hydrogen. Some of the original base remains in the solution.

Acids And Bases I Introduction

Chemistry The Central Science Chapter 16 Section 7

Weak Base Strong Acid Reactions Acids And Bases Ap Chemistry Khan Academy Youtube
0 Comments